Regulatory Process Overview – ACTUAL RESULTS
Numbers of Clinical Trials in Japan
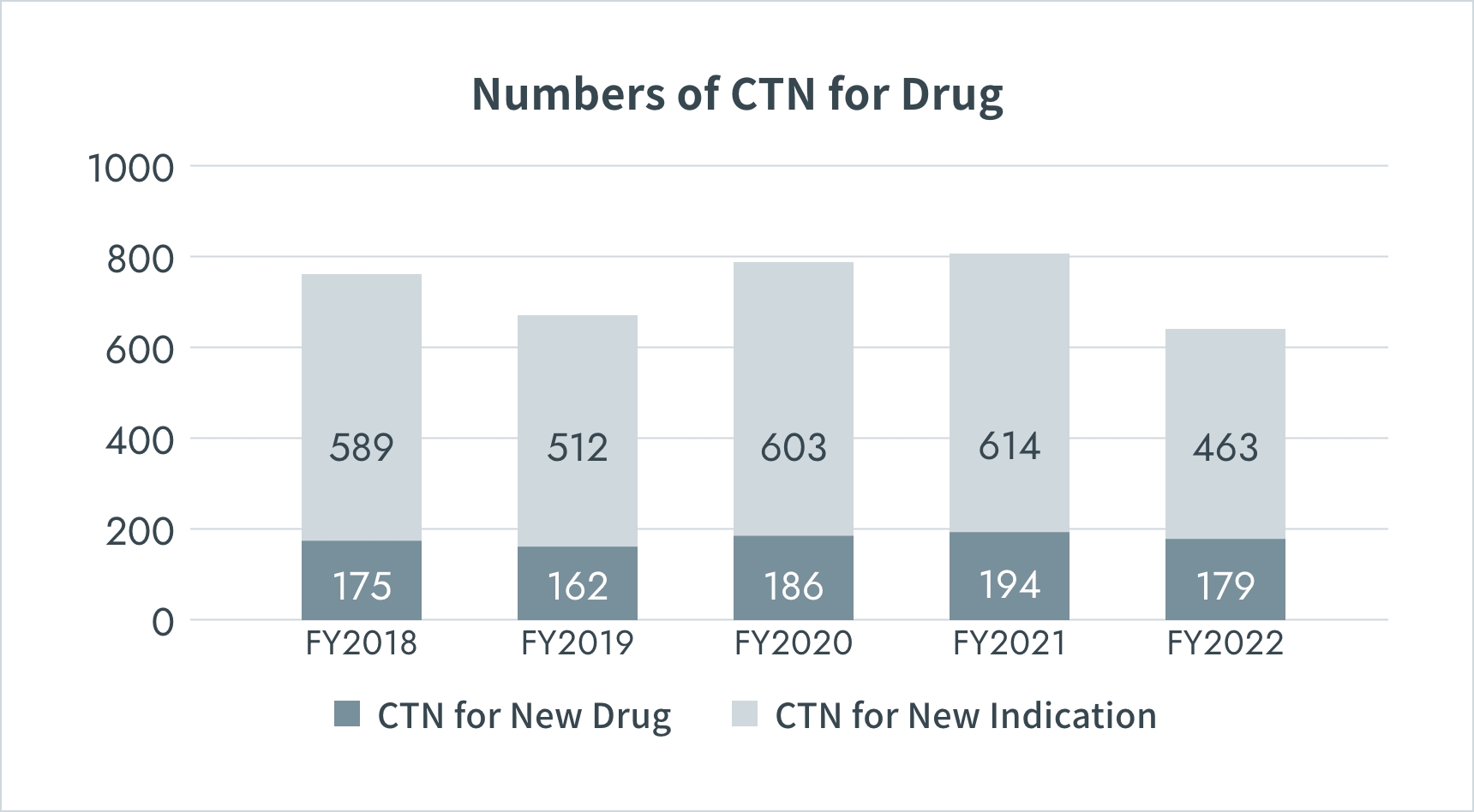
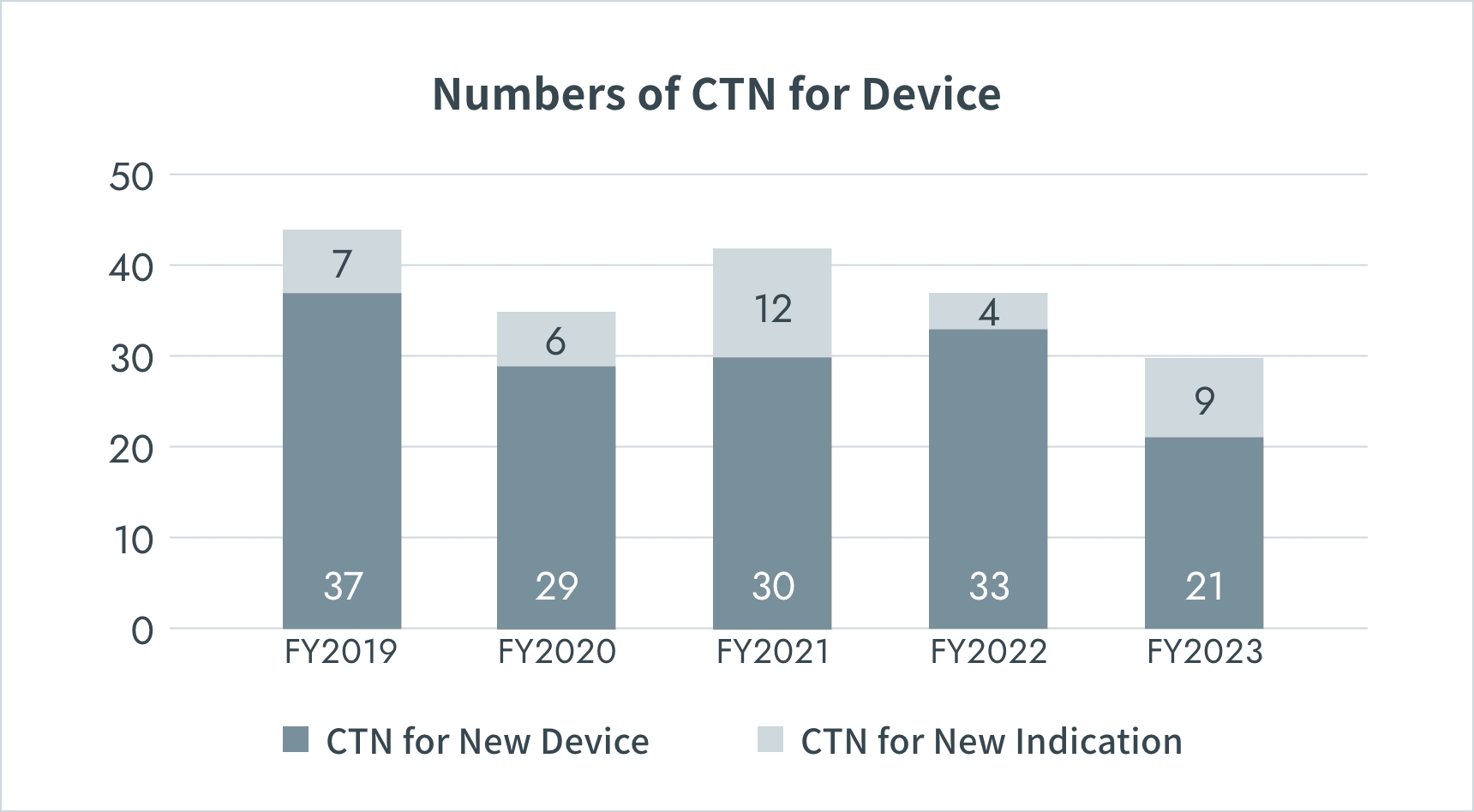
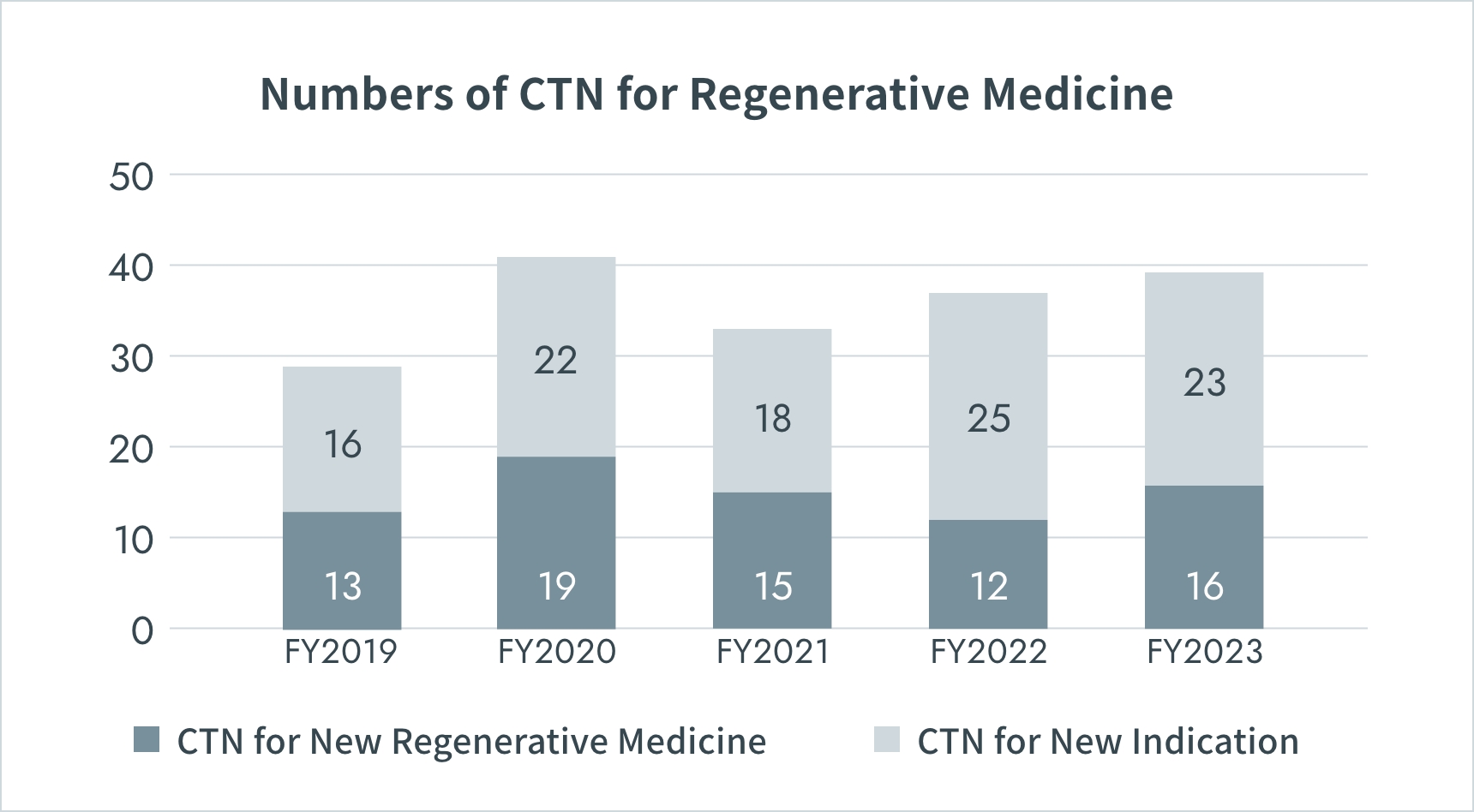
*CTN : Clinical Trial Notification
Source: PMDA(https://www.pmda.go.jp/)
Trend in Number Clinical Trial by Trial Type
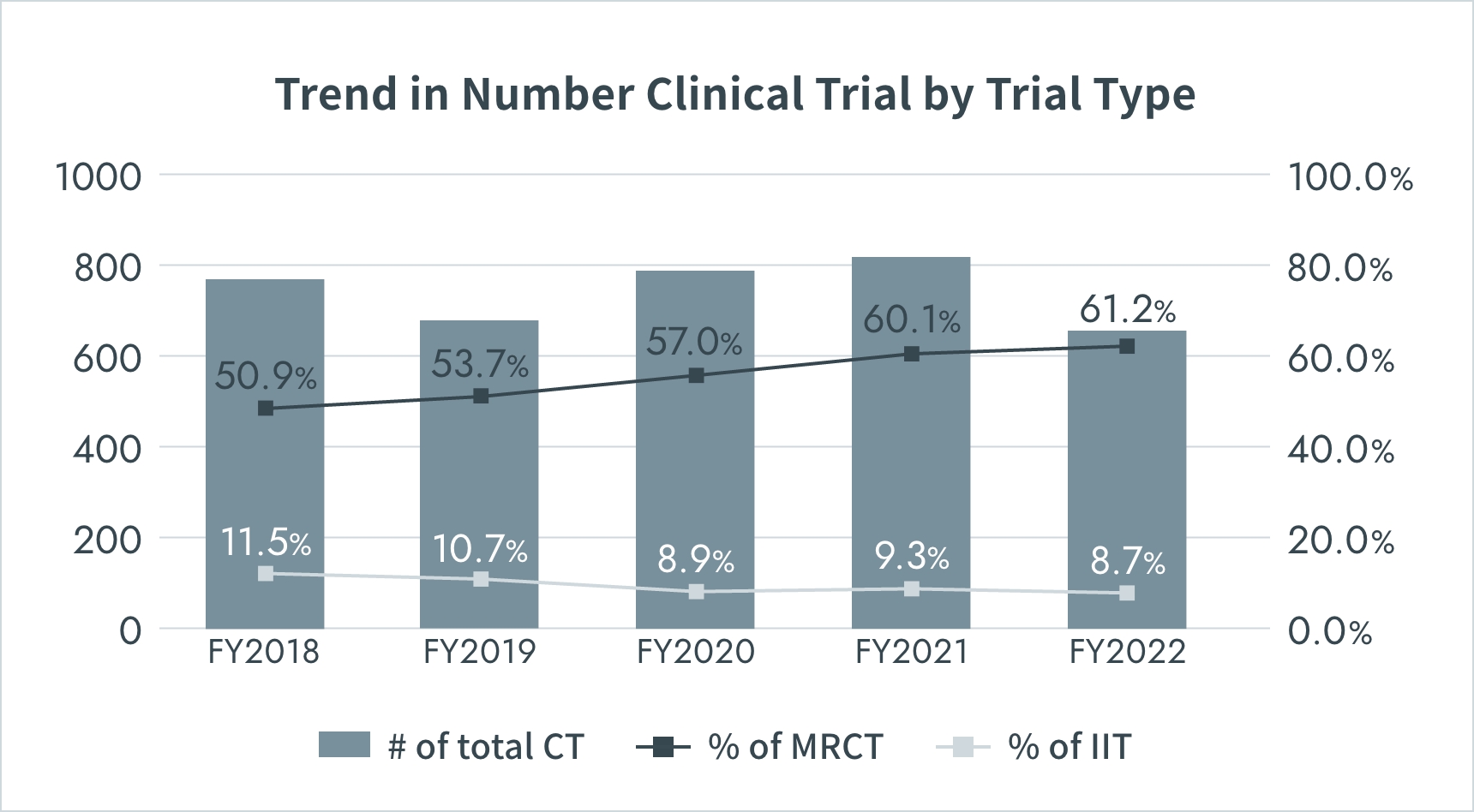
*CT : Clinical Trial
*MRCT : Multi Regional Clinical Trial
Source: PMDA(https://www.pmda.go.jp/)
Numbers of PMDA Consultation
Numbers of Consultation for Drugs and Regenerative Medicines
| Fiscal Year | FY2018 | FY2019 | FY2020 | FY2021 | FY2022 |
|---|---|---|---|---|---|
| Before Phase I Trial Consultation | 41 | 36 | 22 | 31 | 30 |
| Before Phase IIa Trial Consultation | 3 | 10 | 6 | 9 | 5 |
| Before Phase IIb Trial Consultation | 39 | 36 | 39 | 32 | 41 |
| After Phase II Trial Consultation | 154 | 160 | 177 | 185 | 180 |
| Before New Drug Application Consultation | 47 | 62 | 68 | 44 | 55 |
Refer to following link about PMDA Consultation
https://www.pmda.go.jp/english/review-services/consultations/0002.html
Source: PMDA(https://www.pmda.go.jp/)
Numbers of New Drug Application (NDA) and Approval in Japan
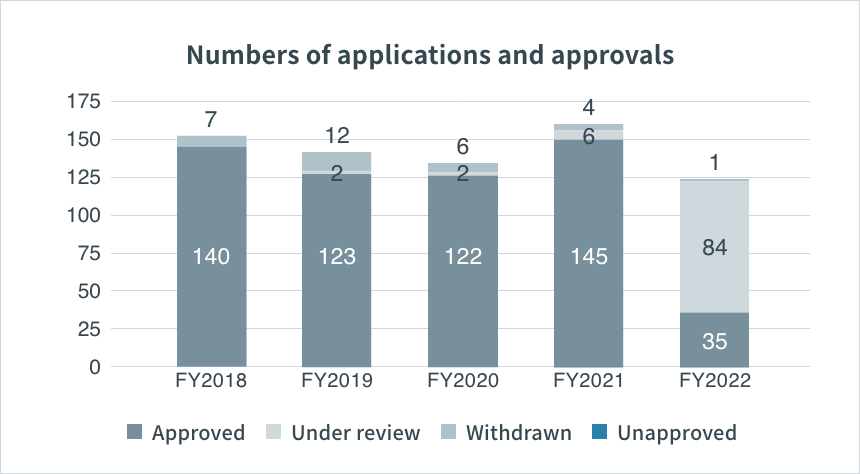
Source: PMDA(https://www.pmda.go.jp/)
